VAD Patient Reported Outcomes
What is the project focus?
Comprehensive assessments of patient well-being (physical, social, and psychological) can help guide clinical care in both the short- and long-term. Given that there is no “gold standard” single assessment tool for young people on ventricular assist device (VAD) support, ACTION uses a combination of tools to evaluate feasibility of PRO administration and evaluate the quality and relevance of the information PROs provide for our patients. We use the Tonic for Health PRO platform to electronically deliver surveys to patients and caregivers directly via text or email.
To learn more, visit My ACTION Education.
Who is impacted?
Heart failure patients implanted with a VAD.
What are we doing to help?
Check out our Taking ACTION on Outcomes that Matter Most to You patient info sheet, or watch the video below to learn more about PROs and how they are used.
What are we doing to help?
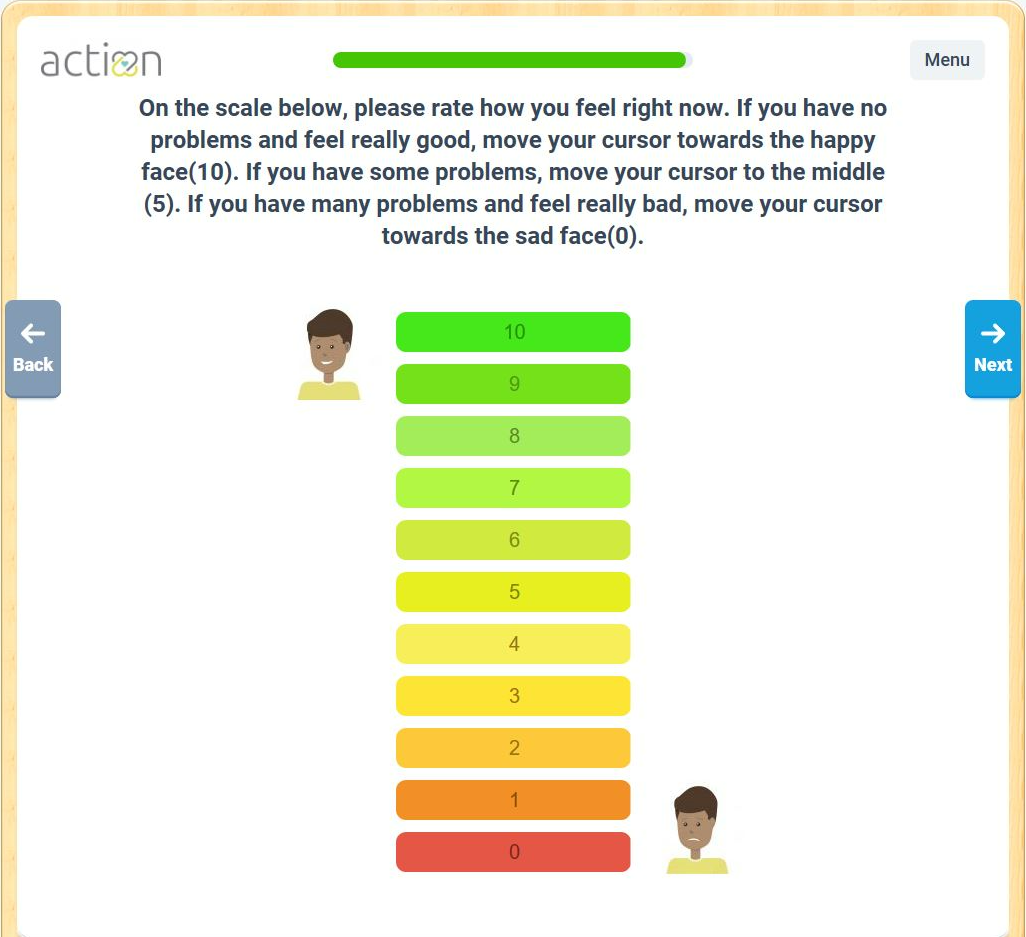
Patients and parents told us how they’re feeling post-VAD implant. 289 surveys were completed from 6/8/21 through 10/17/23. See the Visual Analog Scale results below.
CardioMEMsTM
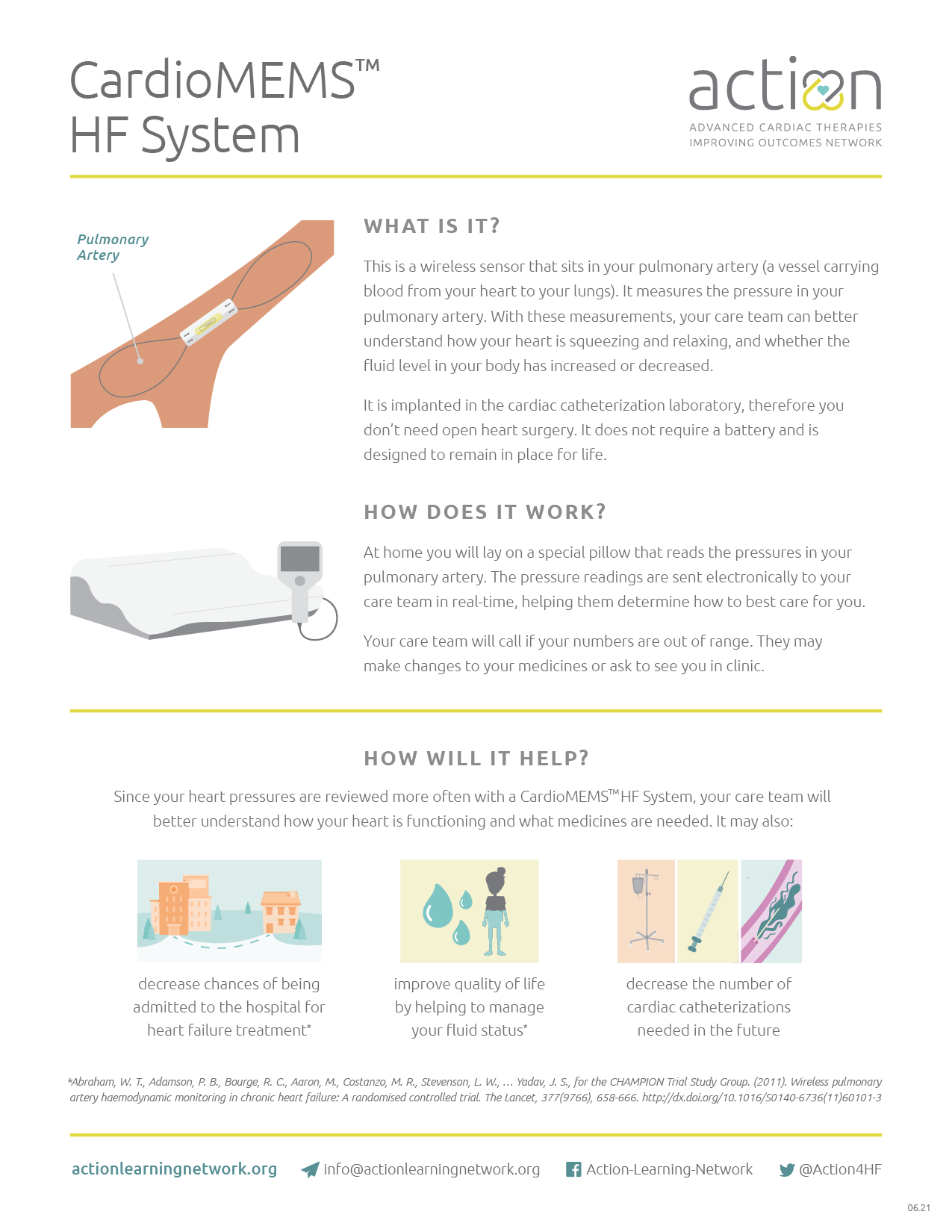
What is the project focus?
The CardioMEMS™ HF System is a FDA-approved implantable monitor for adult patients with heart failure. The studies to date have excluded patients with Congenital Heart Disease (CHD) and of pediatric age from the initial investigations of feasibility of implantation, safety, and clinical outcomes.
The ACTION CardioMEMS™ database will include clinically diverse patients in which the CardioMEMS™ is used off label. We will seek to determine safety and efficacy in these niche populations.
Who is impacted?
All patients who have a CardioMEMS™ device implantation (or attempted implantation) at an ACTION site with the following diagnoses:
- Pediatric patients with congenital or acquired cardiomyopathy and evidence of heart failure
- Pediatric patients with pulmonary hypertension (of any etiology) with established heart failure
- Congenital heart disease patients of any age with evidence of heart failure
- Heart transplant patients with graft dysfunction of any cause resulting in heart failure
What are we doing to help?
Check out our one page summary to learn more about the CardioMEMS™ HF System, including what it is, how it works and how it can help heart failure patients.
Wearables
What is the project focus?
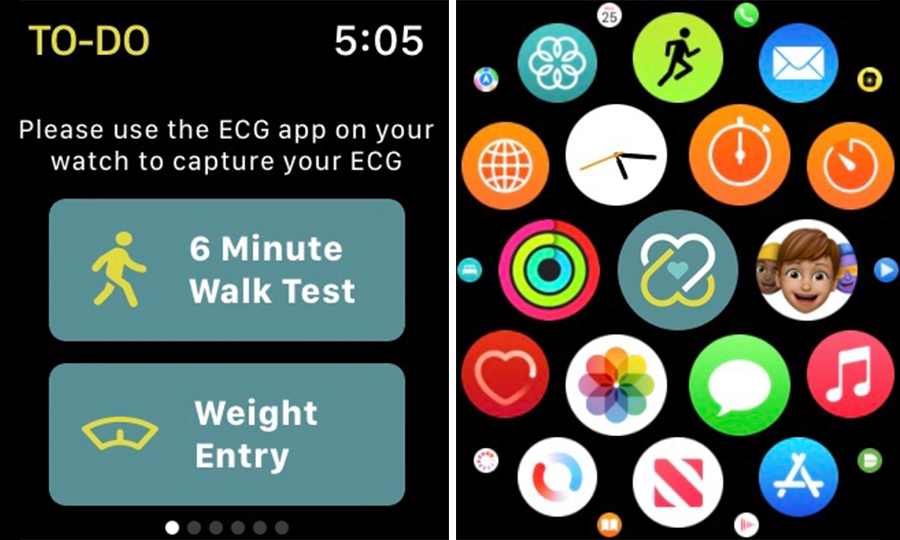
Wearable technology has rapidly advanced in recent years. Devices of reasonable cost and portability are now able to capture a significant amount of physiologic and activity data and may facilitate interventions, presenting opportunities to improve care in heart failure. However, we do not yet know if and how the technology can be optimally adopted and utilized in children with heart failure. We are currently enrolling in a prospective, observational, multi-center cohort study using a custom, pediatric-designed smart watch (Apple Watch) application to demonstrate feasibility and collect actionable biometric health data of children with heart failure.
Who is impacted?
Patients ≥12–19 years old with heart failure.