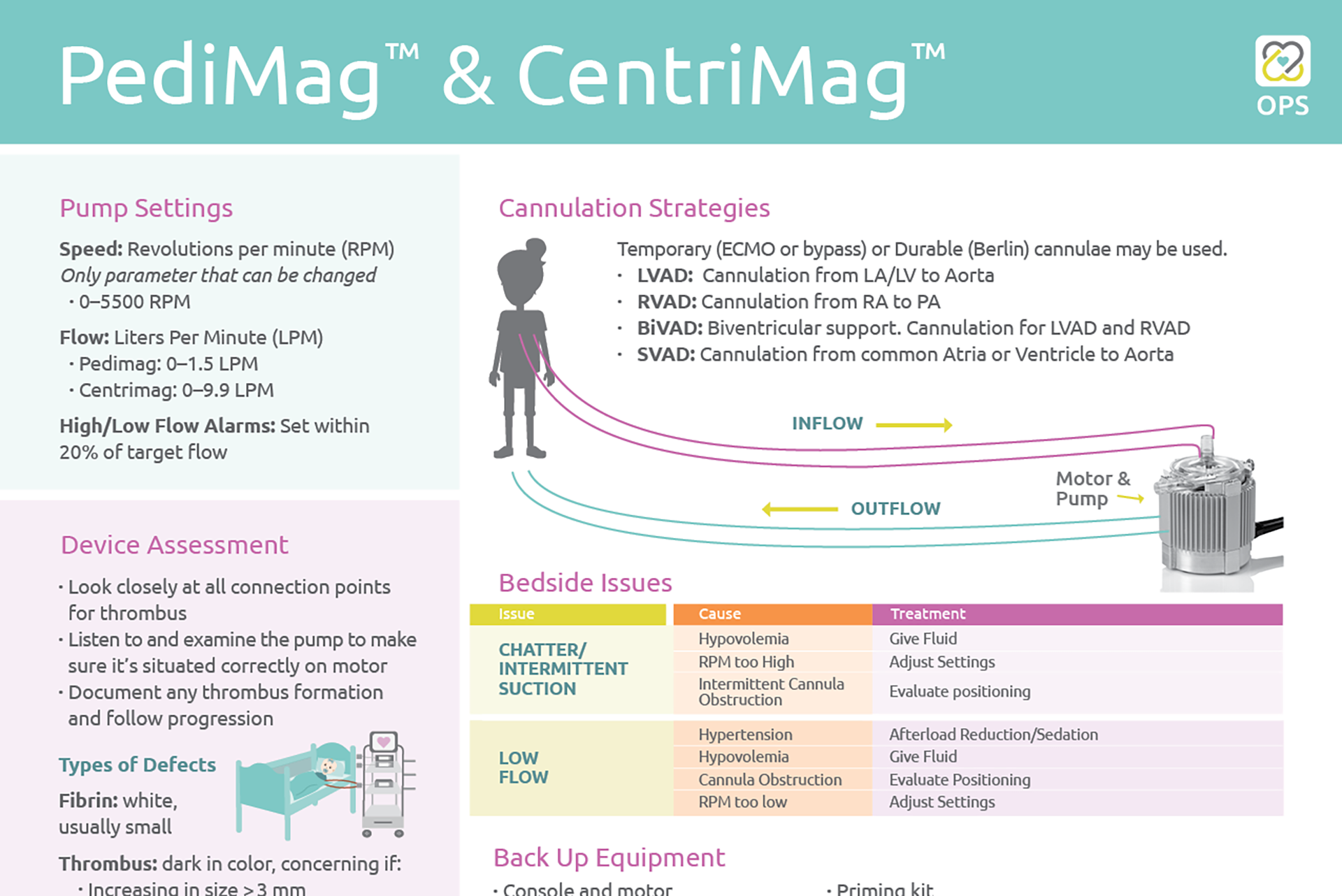
PediMag™ & CentriMag™ OPS
Check out the newest One Page Summary (OPS) featuring the PediMag™ & CentriMag™. This OPS includes an overview of the device’s pump settings, cannulation strategies, system components, bedside issues and backup equipment, […]
PediMag™ & CentriMag™ OPS Read More »